New FDA Approved Drug-Free Treatment for Painful Diabetic Neuropathy

What is Diabetic Neuropathy?
Diabetic Neuropathy is chronic nerve damage in the upper and lower extremities caused by constantly high blood sugars. It is a chronic debilitating condition which can interfere with a person’s sleep, their functionality, and their overall quality of life.
Around 34 million Americans have diabetes (CDC, 2020) and roughly half of all adults with diabetes will suffer from Diabetic Neuropathy in their lifetime (Hicks, 2019). Of those, it’s estimated approximately 2.3 million will suffer from Painful Diabetic Neuropathy (PDN) with no relief by using current treatments and conventional medical management (Schmader, KE., 2020).

What are the Typical Symptoms of Painful Diabetic Neuropathy (PDN)?
Those who suffer from Painful Diabetic Neuropathy typically have daily continuous pain. Typical symptoms of include tingling, numbness, and pain in the extremities. This nerve damage can make patients more vulnerable to falls, burns, infections, ulcers, and long-term complications.
What Treatment Options are there to Relieve Chronic Pain from Diabetic Neuropathy?
Current treatment options aim to relieve the chronic pain that is frequently seen with Diabetic Neuropathy. These treatments often include pain medicines such as Tylenol or Aspirin. NSAIDS such as Ibuprofen or Advil, however, are not recommended for diabetic patients. Some patients with Diabetic Neuropathy are also treated with nerve medications including Neurontin (Gapapentin), Lyrica, and Topomax. When patients don’t respond to these medications, narcotics or opioids are often used.
Although narcotics can lessen pain, they have significant long term consequences including dependence, decreased benefit over time, and abuse. New federal guidelines aimed at reducing opioid misuse and abuse have resulted in many physicians denying or limiting opioid therapy for some chronic pain patients. Physicians are constantly looking to improve clinical and interventional tools to treat complex chronic pain. One of these advancements includes the development of Spinal Cord Stimulation (SCS).
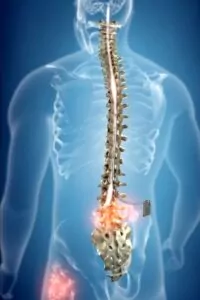
Spinal cord stimulation has been available since the 1960s and uses a device called a Spinal Cord Stimulator to treat chronic pain. This small device is placed on the spinal cord to calm the spinal nerves and suppress the pain responses to the brain. As a result, pain lessens for the patient. Each device is programable to the individual patient’s needs. Additionally, Spinal Cord Stimulation allows for patients to go through a weeklong trial period to test their response to the device before permanent implantation.
There are many types of Spinal Cord Stimulators and the technology and programming options that come with them have made many advancements in recent years. These advancements have shown to provide better pain relief for many chronic pain conditions. Most recently, Spinal Cord Stimulation for Painful Diabetic Neuropathy (HFX™ for PDN) developed by Nevro, received FDA indication and approval to treat Painful Diabetic Neuropathy.
Who is HFX™ for PDN for and what does Spinal Cord Stimulator Surgery entail?
![]()
Patients suffering from Painful Diabetic Neuropathy specifically, who have not responded well to conventional medicine treatments are typically good candidates for a Nevro HFX™ for PDN Spinal Cord Stimulator trial. During this trial period, a temporary version of the stimulator is placed through a needle without undergoing surgery. The patient will then use this device for one week and monitor their pain levels, activity level improvements, and their need for pain medicine. At the end of the trial, the device is removed. Ninety percent (90%) of patients who go through the trial have enough to success to make the decision to move forward with a permanent stimulator (Diabetes Care, 2021).
Once this decision has been made, a permanent spinal cord stimulator can be implanted with a quick outpatient surgery. With any outpatient surgery, the patient is able to go home and recover the same day as surgery. The permanent implant procedure is done through two small incisions. After the incisions are made, a paddle with metal contacts is placed against the spinal cord and a rechargeable battery is placed below the skin.
What is the Average Recovery Time from Spinal Cord Stimulator Surgery?
Moderate activity is limited, and strenuous activity is restricted for approximately six weeks post-surgery. This recovery time ensures that the device is received well, and the body has time to heal. However, despite this dedicated recovery time, many patients can feel pain relief in as little as a few days after their operation.
Recovery is quick with approximately eighty six percent (86%) of patients with Painful Diabetic Neuropathy experiencing substantial, long-term relief after 12-months (JDST, 2021). Additional details of the study can be found here.
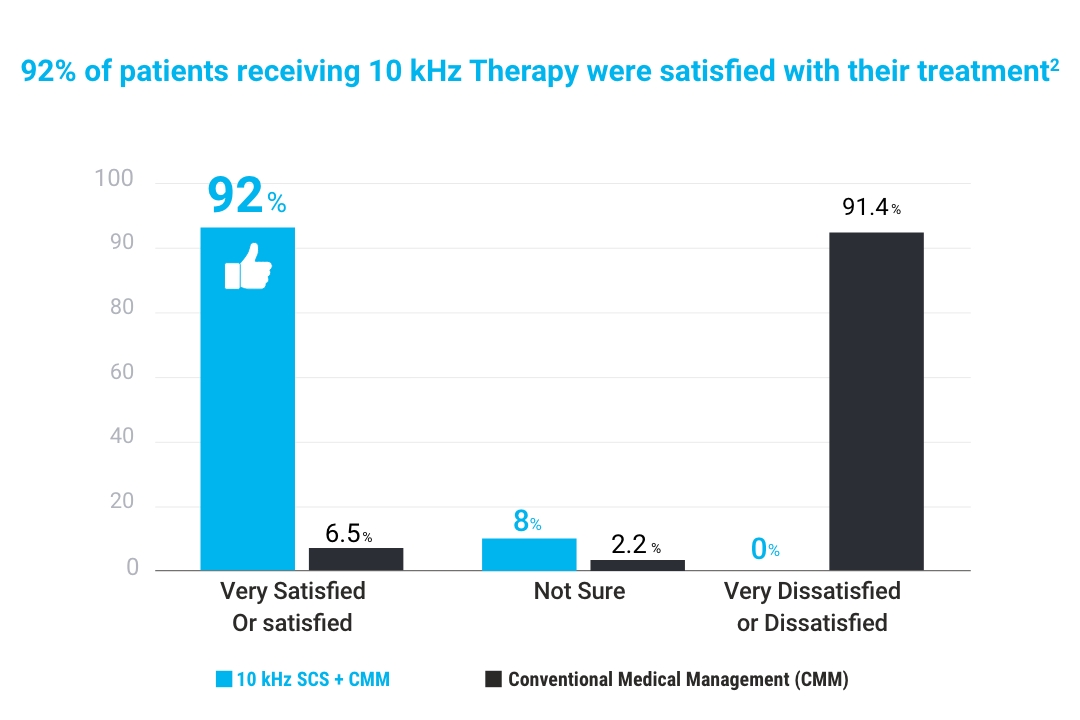
Stay tuned, ongoing studies are showing great success using Spinal Cord Stimulation (SCS) to help treat additional types of Neuropathy such as Peripheral Neuropathy.
Click Here for additional Data on Nevro’s HFX™ for PDN or visit https://www.hfxforpdn.com/

Roger D. Sung, MD
Dr. Sung is a Fellowship-Trained and Board-Certified Orthopedic Surgeon who specializes in Cervical, Thoracic, Lumbar, and Sacroiliac surgery, Microsurgery, and Minimally Invasive Spine Surgery techniques. He also performs complex spine reconstruction using minimally invasive techniques.